Orbital Diagram Of Carbon Before Sp3 Hybridization


Answer to write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization. Write the orbital diagram of carbon before sp3 hybridization.

Please just explain what the orbital looks like. So no, the atom doesn’t have to get excited to 1s2 2s1 2p3 before In the case of sp3 hybridization, say in methane, the carbon s orbital. Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals.

1. orbital makes four, sp3 orbitals in a tetrahedral array.

Molecular Orbital of Methane, CH4. 1.
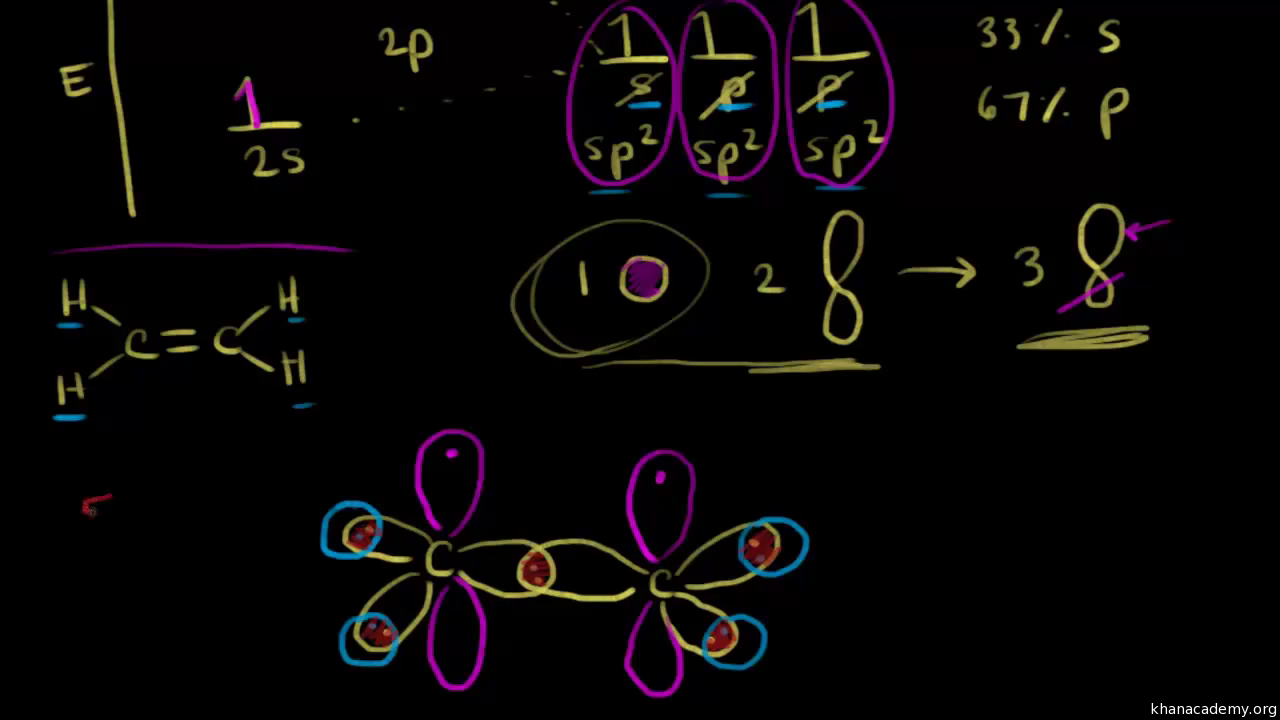
The Lewis structure shows us that the carbon atom makes 4 sigma bonds to hydrogen and has no . Ethene, sp2 hybridization with a pi bond.
1.

The bonds in a methane (CH4) molecule are formed by four separate but equivalent orbitals; a single 2s and three 2p orbitals of the carbon hybridize into four.To account this, sp 3 hybridization before the bond formation was proposed. * During the formation of water molecule, the oxygen atom undergoes sp 3 hybridization by mixing a 2s and three 2p orbitals to furnish four sp 3 hybrid orbitals oriented in tetrahedral geometry.

Because this type of sp hybridization only uses one of the p orbitals, there are still two p orbitals left which the carbon can use. Those p orbitals are at right angles .

Question: Write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization. write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization. Best answer.

86 % (7 ratings) Get this answer with Chegg Study86%(7). Carbon is making 2 s and 2 p bonds to the oxygen atoms.

The 2 s bonds indicate that there are 2 equivalent molecular orbitals formed. To form 2 hybrid molecular orbitals, we need to mix 2 atomic orbitals, an s orbital and a p orbital. The resulting hybrid orbitals are called sp hybrids.

sp 3 Hybridization. The ground state configuration of carbon is 1s 2 2s 2 2px 1 2py 1. The p orbitals are equal in energy and said to be degenerate.

The two singly occupied p orbitals can be utilized for bonding to give methylene CH 2, an unstable free radical (Figure 3).Consider the electron configuration of a carbon atom.?
| Yahoo AnswersConsider the electron configuration of a carbon atom.?

| Yahoo Answers