Energy Diagram Catalyzed Vs Uncatalyzed Reaction


Catalyzed and Uncatalyzed Reaction Pathways. Figure Potential energy diagram of catalyzed vs uncatalyzed reaction pathway.

Potential energy. Energy Diagrams for Catalyzed and Uncatalyzed Reactions.

Page 2. Page 3.

Page 4. Enzymatic Catalysis of a Reaction between Two Substrates.

Page 5. Enthalpy profile for an non–catalysed reaction, last page a typical, non– catalysed reaction can be represented by means of a potential energy diagram. Therefore, only a few collisions will result in a successful reaction and the rate of.

The only difference between a catalyzed reaction and an uncatalyzed reaction is that the activation energy is different. There is no effect on the. Catalysis is the process of increasing the rate of a chemical reaction by adding a substance Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher reaction .

This effect can be illustrated with an energy profile diagram.Catalyzed reaction has a lower activation energy because there is an enzyme present in the reaction. Uncatalyzed reaction has a higher activation energy because there is no enzyme present in the reaction.

Energy Diagrams for Catalyzed and Uncatalyzed Reactions. Models of Enzyme-Substrate Interaction.

Enzymatic Catalysis of a Reaction between Two Substrates. Substrate Binding by Serine Proteases. Uncatalyzed reaction Activation energy Substrate (S) Catalyzed reaction Product (P).
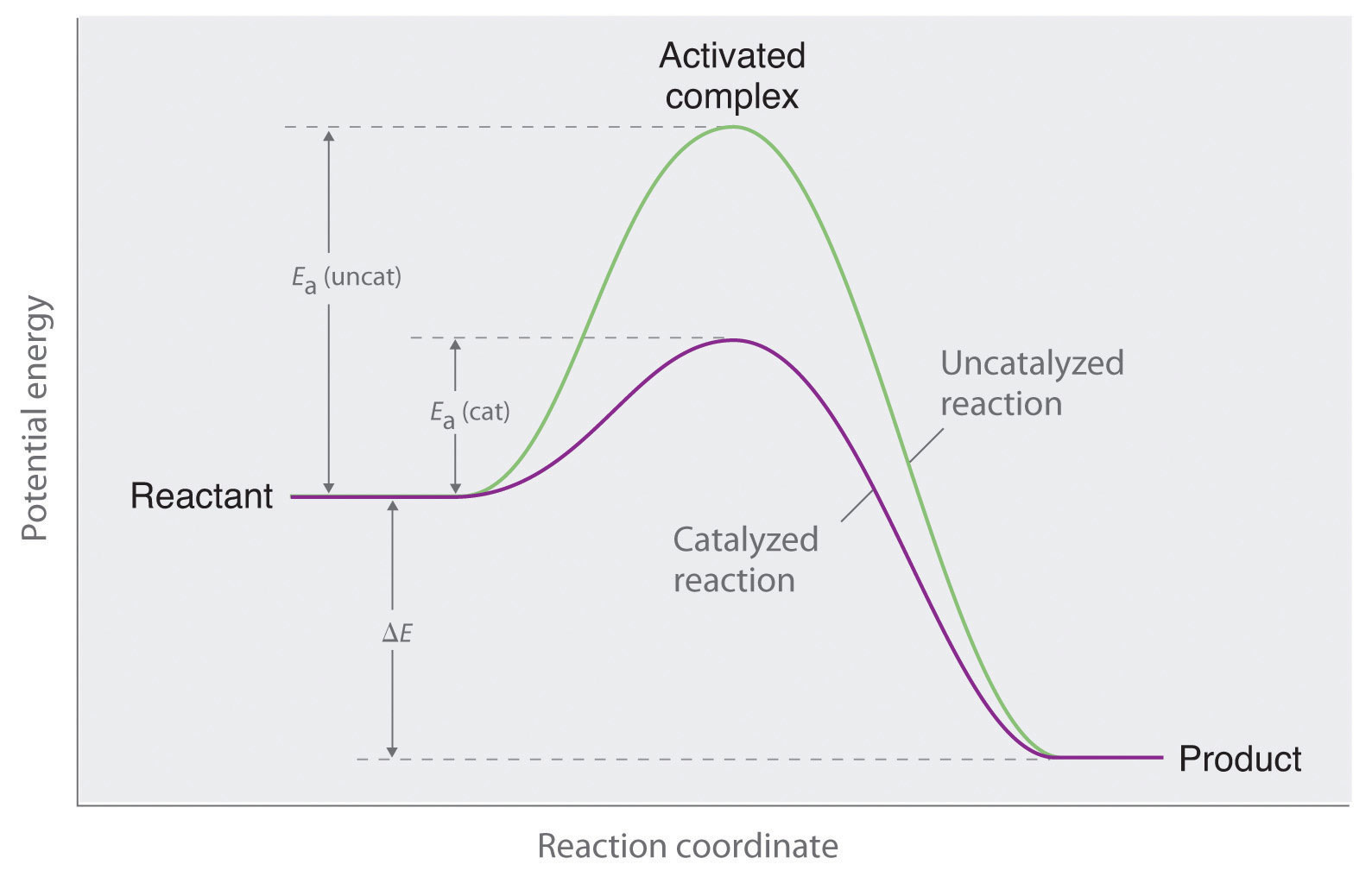
Show transcribed image text Enzymes are important molecules in biochemistry that catalyze reactions. Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction.

Label the energy diagram and answer the question that follows%(1). Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher reaction rate at the same temperature and for the same reactant concentrations.

However, the detailed mechanics of catalysis is complex. c.

Consider the catalyzed reaction. Reactants Products + Energy.

Draw a reaction coordinate diagram for this reaction as above but add the activation energy, E a, for the catalyzed reaction on the appropriate curve in this diagram and label it. This is a bit more subtle since .Types of catalysts (article) | Kinetics | Khan AcademySection The Rate of a Reaction
